Causes of PCOS
Polycystic ovarian syndrome is diagnosed in different ways across the globe. In Europe, the Rotterdam criteria are used and require that a woman presents with two or more signs and symptoms out of three typical observations. This approach ensures that there are stark differences between the range and severity of the syndrome - the venn diagram here is a graphical representation of the heterogeneity of women diagnosed with PCOS in Europe. Unlike many medical conditions causation is not central to the diagnosis which limits how the syndrome is treated - therapy tends to be targeted at symptom relief rather than addressing the underlying cause(s).
In the USA the approach is quite different. Polycystic ovaries are only seen in about 2/3 of women diagnosed with PCOS so it is not a very reliable sign for diagnostic purposes. The National Institute of Health (NIH) recommends that ovarian morphology is not used as part of the normal diagnostic regime. Recently, it has been found that 65-80% of women with PCOS are insulin resistant, irrespective of BMI; and in the group of women with PCOS whose BMI is above 30 the prevalence is around 95%. Clearly, the link between PCOS and insulin resistance is far more significant than the appearance of the ovaries that gives the syndrome its name. And that is where the therapeutic role of Replenitol is targeted.
Why is insulin resistance important and how can it affect so many women in so many different ways? We have spent many years studying this conundrum and have trawled all the published studies in the field over the past 170 years. By going back to the very beginning of documented research in the field we have been able to draw out the relevant findings and assess them in the light of the accumulated knowledge. Our findings are quite remarkable and have produced a syndrome model that is logical, simple and predictive. More importantly, it allows us to target treatment at the underlying cause of PCOS in the majority of cases and to understand why it works so well.
Women with PCOS excrete 2 to 3 times the normal amount of myo-inositol
The excessive rate of excretion by the kidneys of a key metabolite involved in normal insulin signalling can lead to insulin resistance. We now know that 65-80% of all women with PCOS are insulin resistant so we have based our model on this core observation. The link between excessive myo-inositol excretion, known as inosituria, and insulin resistance was made by a PhD student in Nuremberg in the 1840s who noted that people with diabetes excreted 5-6 times the normal amount of myo-inositol. There is a lack of follow-up studies until the 1950s and there has been a further hiatus until very recently. How can inosituria lead to PCOS?
Inosituria causes insulin resistance in PCOS
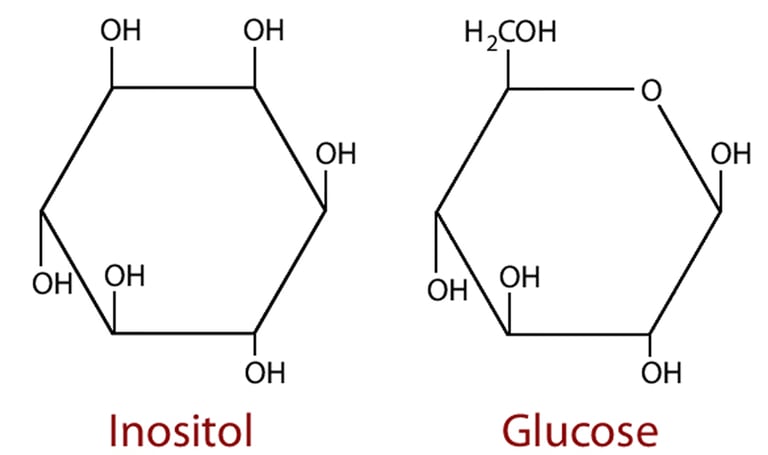
Myo-inositol is produced in the kidneys from glucose in a 2 stage pathway so the two molecules retain significant structural similarities and share identical empirical formulae. Their handling in the kidneys is also similar as both pass into the filtrate and are actively reabsorbed in the proximal tubule by specific carrier proteins. The receptor for myo-inositol is known as SMIT2 (Sodium Myo-Inositol Transport type 2) and glucose is reabsorbed by SGLT2 (Sodium GLucose Transport type 2. Under normal circumstances each transport is responsible for only reabsorbing its specific eponymous target.
Incidentally, SMIT2 is also classified as SGLT6 which gives a further clue to how similar the transports are and why SMIT2 can also reabsorb glucose.
How can inosituria precipitate insulin resistance?
Positive Feedback Loop
Under hyperglycaemic conditions circulating myo-inositol is reduced. Myo-inositol is integral to at least 5 intracellular insulin second messengers so the resultant deficiency suppresses the normal cellular response to insulin - i.e. myo-inositol deficiency can cause insulin resistance.
The reduced blood glucose-lowering capacity increases the duration of hyperglycaemic conditions leading to even greater depletion of circulating myo-inositol which exacerbates insulin resistance driving a vicious cycle or positive feedback loop.
Could supplementing the deficiency break the positive feedback loop and help women with PCOS?
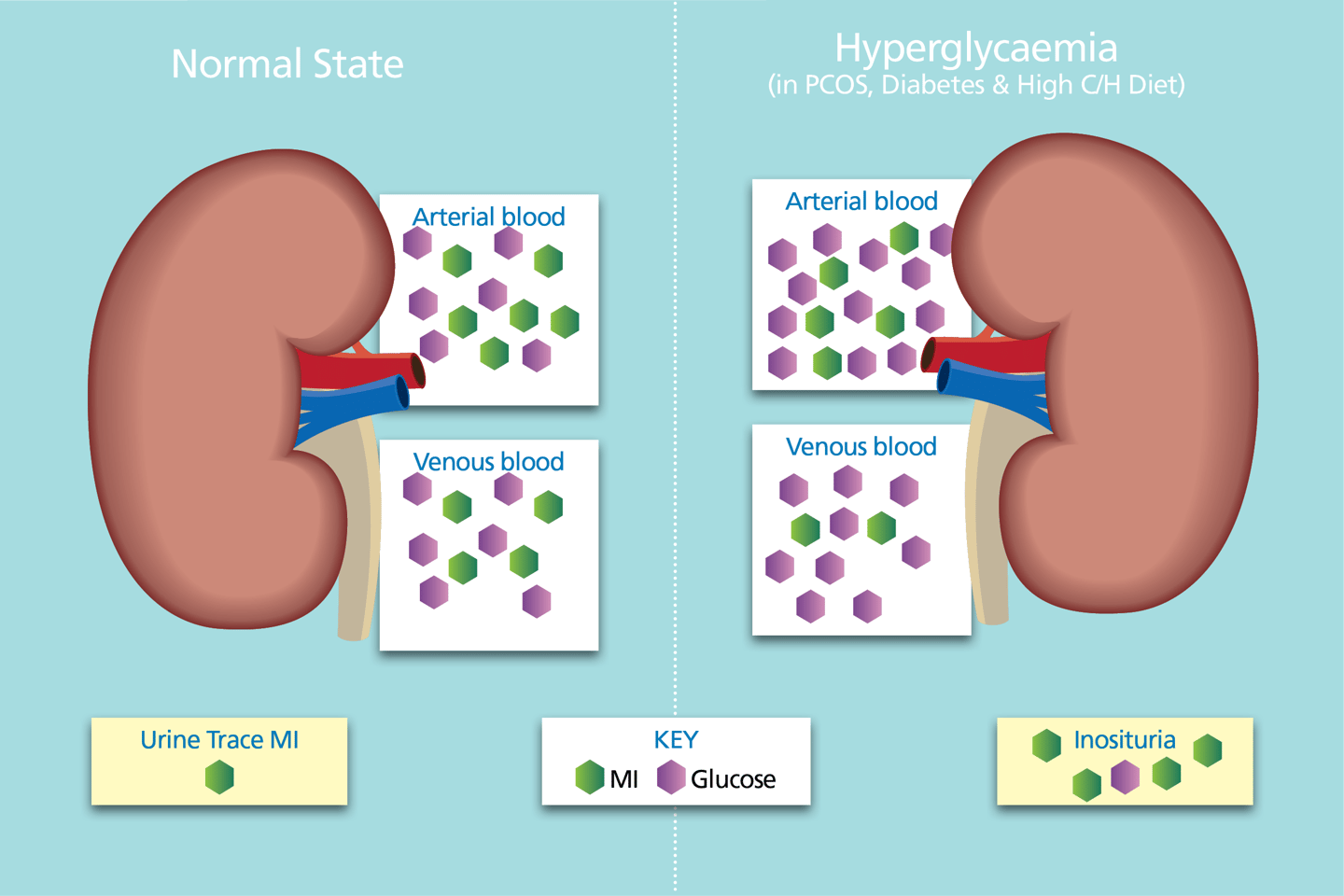
Arterial blood is filtered by the kidneys.
Almost 100% of glucose and myo-inositol enter the filtrate - both are actively reabsorbed.
Under normoglycaemic conditions, the activity levels of the sodium myo-inositol transport type 2 (SMIT2) co-transporters is normal and ≈100% is reabsorbed.
Glucose is a precious resource - maintaining circulating glucose is a priority.
In hyperglycaemia, SMIT2s become inundated with glucose thereby reducing the reabsorption capacity of SMIT2 activity for myo-inositol reabsorption.
More myo-inositol is excreted as a result.
In T2D and PCOS, urinary myo-inositol is typically 4-6x & 2-3x higher than the normal state. (6,7,8,9)

